|
|
|
|
|
|
|
Process and patterns of snow melt |
|
Let the snow melt. How much energy per gram ice/water is needed to change the aggregate state from snow/ice to water? |
|
| The phase change from solid snow to liquid water requires a comparatively high amount of energy. In the animation shown here, you can see how much energy per gram ice/water is needed at constant temperature to realise a phase change from solid to liquid. As the incoming radiation supplies enough energy, snow grains begin to melt. |
|
Compare the molecular structures of water and ice. |
||
|
Chemistry of meltwater The chemistry of the melt-water reflects the composition of elements and ions in the snow. The concentration of species and hence the pH of melt-water may change considerably during the melting process.The majority of the ionic content is released with the earliest fraction of meltwater. At first, the most weakly bound ions are released, successively followed by more strongly bound ions. This succession in chemical species of high concentration is called ionic pulse. Ionic pulses of snow meltwater probably play an important role in supplying a considerable amount of nutritive elements to the alpine plants at the onset of the growing season. In general snow and meltwater are much more "dilute" then meteoric waters (higher concentration of solutes (40-120 µS)) but both are dominated by calcium and bicarbonate. |
|
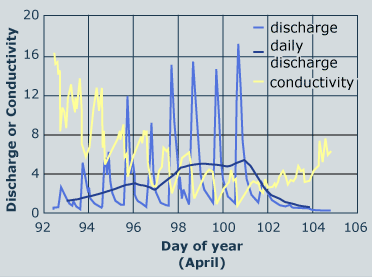
3 - The concentration of ions is highest when the melting process begins. Ionic pulse observed in a 1 x 1 meter square lysimeter at Mammoth Mountain, California study site, 1989. Source: Bales et al., Hydrol. Proc. 7:389, 1993 |
Often big regions in lower elevations depend heavily on the seasonal snowpacks in alpine regions (water and energy). This water resource is sensitive to climate change and pollution. Acid deposits and other chemical loading will be transported very quickly by melt water which has not been filtered as long or as well as rock water. Other conditions (prevalence of thin, sparse soil derived from granitic rocks) reduce the capacity of many alpine catchments to neutralize acid precipitation. Additionally physicochemical processes in the snowpack result in flushing of ionic solutes from the pack at the onset of snow melt. Hence, concentration of ions depends on the quality and quantity of snow fall. |
29 August 2011 |
||
| |
||